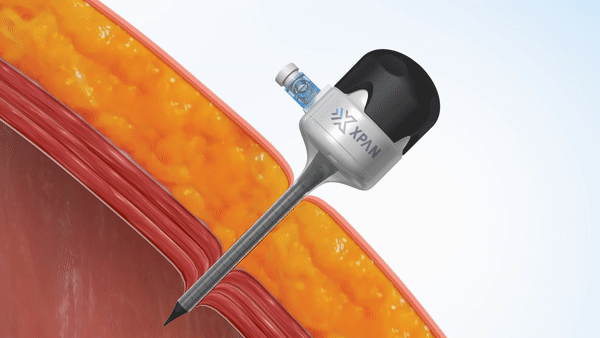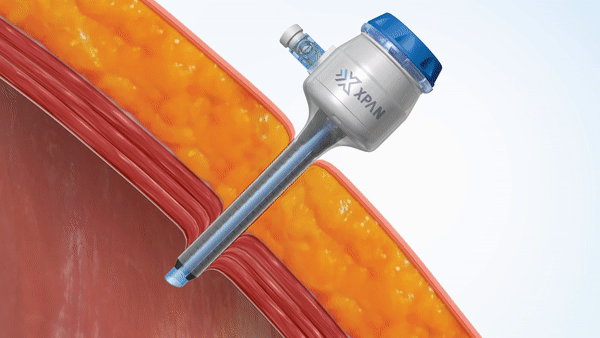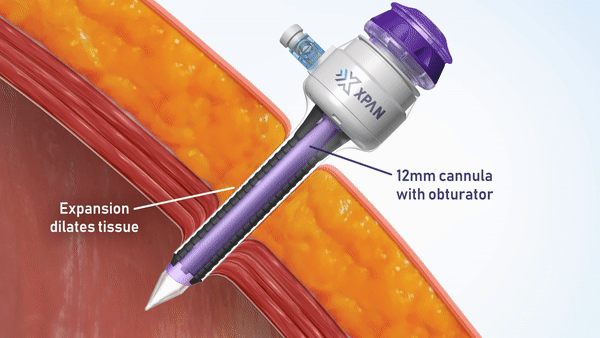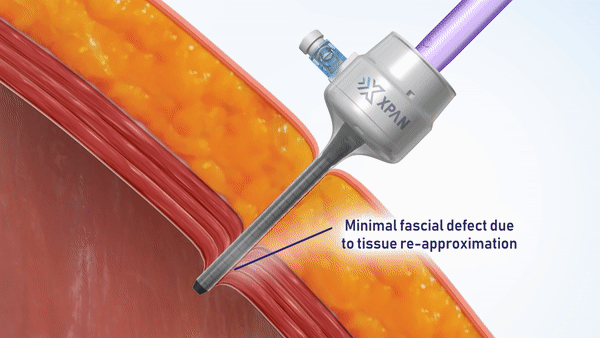
H2i venture Xpan recently announced two exciting milestone accomplishments in a Business Wire press release. The team announced both the securing of FDA 510(k) clearance for their Xpan™ Universal Trocar System, and the successful completion of human clinical procedures using their innovative device. Xpan’s first-of-its kind medical device is a radially expanding trocar that can be tailored by surgeons during laparoscopic procedures to accommodate 3 mm up to 12 mm instruments. This enables surgeons to use minimally invasive techniques and instruments, reduces complications and improves post-op outcomes.
Congratulations, Team Xpan!
Read more about these remarkable milestones here!

